Background:
The use of a cryoprotectant combining both intracellular and extracellular protectants (DMSO+HES) permits large volume hematopoietic stem cell (HSC) cryopreservation in mechanical freezers at -80C without the development of clumping after thawing (Stiff et al, Blood 1983. PMID: 6187518) and was recently utilized for allograft cryopreservation during the COVID-19 pandemic. However, there are competing methods for cryopreservation. Many centers are utilizing vapor phase liquid nitrogen (VPLN) storage protocols with DMSO alone instead. While HSC stored with DMSO alone in VPLN have shown to remain viable after long-term storage (Makhani et al, TCT 2022. PMID:3524877), this has not yet been proven for DMSO+HES stored HSCs at -80C. Here we report on second autologous hematopoietic stem cell transplants (AHSCT2) for relapsed multiple myeloma (RRMM) performed > 2 years after cell storage began at - 80C to determine if there were differences between engraftment and outcomes between initial and long-term storage in DMSO+HES at -80C.
Methods:
We searched our own institution's records from 1990-2023 to find patients who received two separate AHSCT for MM after only one cell collection prior to first AHSCT (AHSCT1). Prior allogeneic transplants patients were excluded. The primary outcome was neutrophil and platelet engraftment times. Secondary outcomes were progression free survival (PFS) and overall survival (OS). We reviewed patient characteristics, disease state prior to both transplants, engraftment kinetics for both, and survival outcomes. Outcomes were analyzed by Kaplan Meier (KM) and longrank test where applicable, and median PFS (mPFS) and median OS (mOS) were determined by reverse KM. Differences in engraftment were compared using the Kruskal-Wallis test.
Results:
35 patients met inclusion criteria, of whom 57% of patients were male. Median age at AHSCT1 was 56.3 years and at AHSCT2 was 60.1 years. Median follow up was 8 years from second transplant and 12.3 years from stem cell collection. Median time from stem cell collection to AHSCT1 was 11 days (range 3-39 days) and to AHSCT2 was 4.6 years (range 2.2-15.8 years).
Prior to AHSCT1, 65% of patients were in PR, 20% in VGPR, and 14% in CR. Prior to AHSCT2, 44% were in PR, 38% in VGPR, and 18% in CR. 80% and 83% of patients were conditioned with melphalan alone in AHSCT1 and 2 respectively. Median CD34+ doses were 5.1x10^6/kg and 4.2x10^6/kg (p=0.028) for AHSCT1 and 2 respectively.
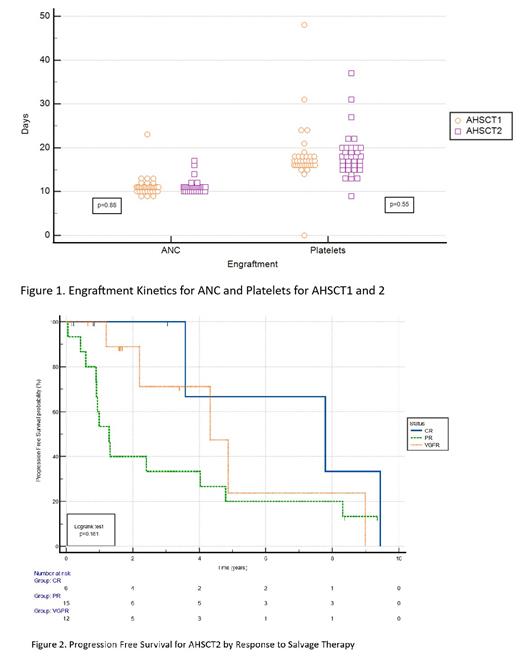
There was no statistical difference between the engraftment kinetics of the first or second transplant with a median ANC engraftment time of 11 days for both groups (p=0.88), and median platelet engraftment of 17.0 and 17.5 days (p=0.55) for AHSCT1 and AHSCT2 respectively. Between categories of cells stored between 2-5 years and >5 years, there was no statistical difference in ANC (p=0.85) or platelet engraftment (p=0.29).
mPFS from AHSCT1 for the overall study was 3.7 years and for AHSCT2 was 3.6 years. The mOS was 4.8 years from AHSCT2. There was a statistical difference in 2-year PFS between ASHCT1 and 2 at 86% and 62% (p=0.0001) respectively. The mPFS differed by disease state prior to AHSCT2 dramatically; those in CR had mPFS of 7.8 years, those in VGPR 4.3 years, and those in PR 1.3 years.
Discussion:
Our results suggest that HSC stored long-term at -80°C using the DMSO + HES combination does not adversely affect product quality even for cells stored up to 15.8 years. Furthermore, our method is simpler, more cost-effective and poses no risk to the working environment when compared to current freezing method where VPLN storage is mainly used. This is particularly important to expand access to cryopreserved HSC for centers and countries where resources are more limited.
These data also add evidence to the efficacy of AHSCT2 as we saw a 2-year PFS of 63% for these patients, which compares favorably to 50% 1-year PFS in AHSCT2 noted in the large CIBMTR retrospective by Dhakal (Leukemia 2021, PMID 32747684). In addition, some patients benefit greatly, best seen in those achieving a CR prior to AHSCT2 who here had a mPFS of 7.8 years. This is an important selection criteria we now evaluate when considering AHSCT2 in RRMM. AHSCT2 is cost effective and compared to recently approved CAR-T and bispecifics is likely a more economical approach in select patients relapsing after long remission following AHSCT1 who achieve deep responses to salvage therapy.
Disclosures
Tsai:Bristol Myers Squibb: Speakers Bureau; Jazz Pharmaceutical: Speakers Bureau. Stiff:Eisai: Research Funding; Incyte Corp: Research Funding; Gamida Cell: Research Funding; Macrogenics: Research Funding; AtaraBiotherapeutics: Research Funding; Takeda: Research Funding; Amgen: Research Funding; CRISPR: Consultancy.